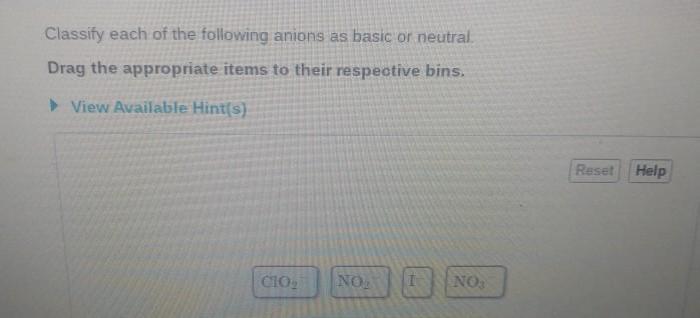
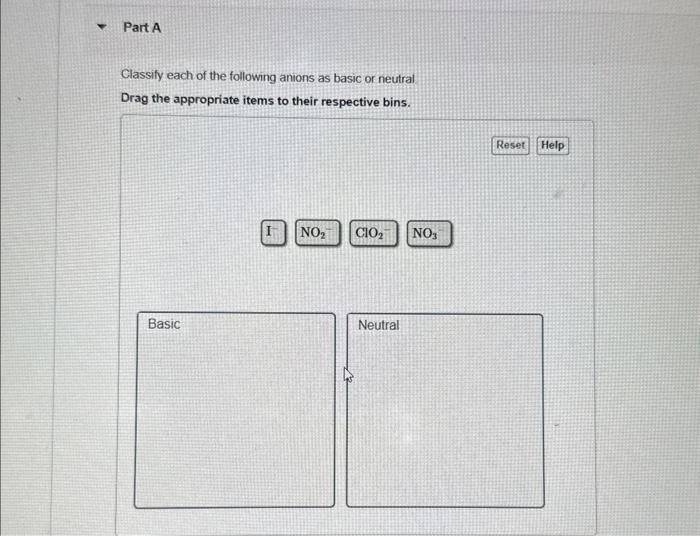
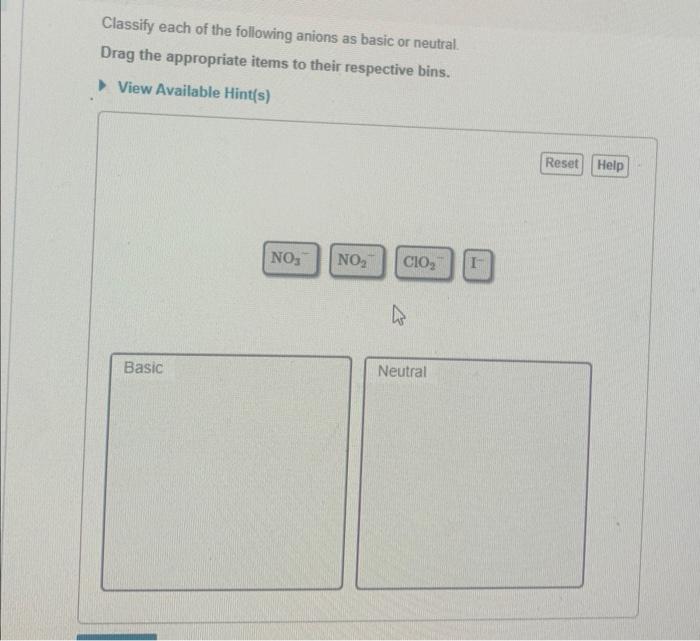
Classify each of the following anions as basic or neutral. This comprehensive guide delves into the concept of anions and their properties, exploring the methods used to classify them and providing examples of both basic and neutral anions. By understanding the classification of anions, we gain insights into their behavior and applications in various scientific fields.
1. Classify Anions as Basic or Neutral: Classify Each Of The Following Anions As Basic Or Neutral.
Anions are negatively charged ions formed when an atom or group of atoms gains one or more electrons. They play crucial roles in various chemical reactions and have distinct properties that influence their behavior.
Anions can be classified as basic or neutral based on their ability to release hydroxide ions (OH-) in aqueous solutions. Basic anions are those that can produce OH- ions, while neutral anions do not.
1.1 Basic Anions
Basic anions are formed from weak acids and have the ability to accept protons (H+ ions) in aqueous solutions. They react with water to form hydroxide ions, increasing the pH of the solution.
- Acetate (CH3COO-)
- Carbonate (CO32-)
- Hydrogen carbonate (HCO3-)
- Hydroxide (OH-)
- Cyanide (CN-)
1.2 Neutral Anions, Classify each of the following anions as basic or neutral.
Neutral anions are formed from strong acids and do not have the ability to release hydroxide ions in aqueous solutions. They do not react with water and have no effect on the pH of the solution.
- Chloride (Cl-)
- Bromide (Br-)
- Iodide (I-)
- Sulfate (SO42-)
- Nitrate (NO3-)
Frequently Asked Questions
What is the significance of classifying anions?
Classifying anions helps determine their chemical properties, reactivity, and behavior in different environments. It enables researchers to predict the outcome of chemical reactions and design materials with specific properties.
How can I apply anion classification in my research?
Anion classification is valuable in various research areas. For example, in environmental science, it helps assess water quality and design remediation strategies. In biology, it aids in understanding enzyme mechanisms and drug interactions.